| Articles on
Pharmacy Design
Clean Spaces - A look at the revised and reissued USP
797 pharmacy design regulation, © William N. Bernstein,
ACHA, AIA
A revised
version of the United States Pharmacopoeia (USP) 797
“Pharmaceutical Compounding—Sterile Preparations”
regulation has been issued this year. All health
facilities that prepare compounded sterile preparations (CSPs)—pretty
much all hospital pharmacies and many off-site
pharmacies—should be aware of this new regulation, whether
or not it is adopted within the facility’s particular
state.
The 2008
version of the USP 797 regulation is the second formal
issue of the regulation by USP. The first formal issue was
in 2004. The new 2008 version contains important, new
requirements that differ substantially from the
now-superseded 2004 version.
Minimize
risk, maximize safety
The
regulation is named after a chapter published by the
United States Pharmacopoeia-National Formulary (USP-NF).
Starting with the first edition of USP 797 in 2004, and
carrying over into the current edition, USP 797 spells out
a comprehensive quality system for the design and
operation of sterile compounding areas and their support
spaces. The intent of USP 797 is twofold: to minimize the
risk of contamination of CSPs as well as to maximize the
safety of the environment for staff working in these
areas.
|
Click image to
download larger version.
|
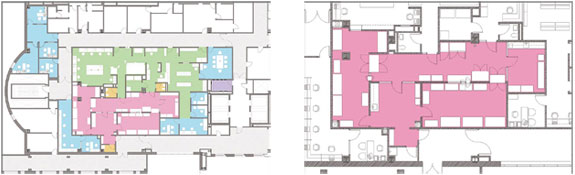
|
Left The overall pharmacy plan for Roswell Park
Cancer Institute.
Right Detail of
the anteroom and compounding rooms.
|
Looking now at
the details of the 2008 edition of USP 797, the general
intent of the regulation remains the same. In the words of
the first sentence of the 2008 regulation: “The objective
of this chapter is to describe conditions and practices to
prevent harm, including death, to patients” that could
result from improper compounding practices done in an
improper environment.
The standards
of the regulation are intended to apply to “all persons
who prepare CSPs and all places where CSPs are prepared
(e.g., hospitals and other health care institutions,
patient treatment clinics, pharmacies, physicians’
practice facilities and other locations and facilities in
which CSPs are prepared, stored and transported).”
The regulation
classifies five types of compounding environments:
low-risk CSPs with 12-hour beyond use dating (BUD),
low-risk, medium-risk, high-risk and immediate use. The
classification of the compounding environment influences
its required characteristics, so it is important that
clients determine their compounding risk as part of the
initial planning of any USP 797-compliant project.
Design and
construction
There
are 25 sections in the 2008 regulation, as well as four
appendices, and the regulation has far-reaching goals in
terms of the design and operation of a facility involved
with CSPs. However, the focus of this article is the
design and construction of a USP 797-compliant
environment, which is addressed in the section of the
regulation entitled: “Facility Design and Environmental
Controls.”
A good place
to start is the two key areas of the 2008 requirements
that differ substantially from the 2004 requirements. They
include the following:
ISO standard for buffer area.
In the 2008 standard, the buffer area
(the room or area where the compounding work is done) must
meet a minimum ISO 7 cleanroom standard (equivalent to a
Class 10,000 cleanroom). In the 2004 standard, the buffer
area only had to meet ISO 8 (the lower the ISO number, the
higher the standard).
ISO standard for ante area.
In the 2008 standard, the ante area
(the room or area that one passes through on the way to
the buffer area) must meet a minimum ISO 8 cleanroom
standard (equivalent to a Class 100,000 cleanroom). In the
2004 standard, the area did not have to meet an ISO
standard.
A central
element in a USP 797-compliant facility is the primary
engineering control (PEC). The PEC is the piece of
equipment, such as a laminar airflow workbench or
biological safety cabinet, where the actual aseptic
compounding activities are performed.
The PEC is
typically located within the buffer area, and the internal
work chamber within that equipment that must equal ISO 5.
In the low-risk level CSP with 12-hour BUD section, the
PEC is permitted to be located in a segregated area
(unclassified space). An example of this type of facility
configuration would be a satellite pharmacy.
In the
planning process, attention should be given to placing
these devices “out of the traffic flow and in a manner to
avoid disruption from the HVAC system and room
cross-drafts.” Selecting that piece of equipment—or
pieces, as larger compounding rooms may have several—is an
important early stage decision in the pharmacy planning
process.
Also central
to the design of a USP 797-compliant facility is the
physical relationship between the buffer area and ante
area. This physical relationship hinges around hazardous
versus non-hazardous compounding as well as the
classification of the nonhazardous compounding room as
low, medium or high risk (hazardous compounding rooms are
always classified as high risk).
There must be
a physical separation (i.e., wall and door) between the
buffer area and ante area in high-risk environments. No
physical separation is required in the other facility
classifications, however, there are daunting HVAC
requirements that come into play for “open” facilities (no
wall/door between buffer and ante areas), as we shall see
shortly.
Many
pharmacies compound substances, such as chemotherapy
drugs, referred to as hazardous drugs as defined in the
National Institute for Occupational Safety and Health (NIOSH)
publication on “Preventing Occupational Exposure to
Antineoplastic and Other Hazardous Drugs in Health Care
Settings.” These drugs are extremely toxic and dangerous
to staff if not transported, stored and handled properly.
Hazardous compounding should be done in a separate
hazardous buffer area. Some hospital pharmacies will have
two buffer areas—a hazardous buffer area and a
nonhazardous buffer area—often served by one common ante
area. One point worth mentioning here is that any ante
area serving a hazardous buffer area must be a minimum of
ISO 7 (because the air from the ante area is being drawn
into the hazardous buffer area).
For a
nonhazardous buffer area, where there is a separation
between the buffer area and the ante area, the buffer area
should be pressurized to be positive relative to the ante
area (i.e., the air should flow from the buffer area to
the ante area, so the cleaner buffer area environment is
not contaminated by the less clean ante area environment).
The regulation specifies that nonhazardous buffer areas
that have a physical separation should have a minimum,
differential, positive pressure of 0.02- to 0.05-inch
water column. Within the nonhazardous buffer area, the PEC
is a device that recirculates air within the room.
All hazardous
buffer areas must have a separation between the buffer
area and the ante area. The buffer area should be
pressurized to be negative relative the ante area (i.e.,
the air should flow into the buffer area from the ante
area, so the potentially more hazardous air of the buffer
area is contained and it does not contaminate the cleaner
ante area environment). The regulation specifies that for
these hazardous buffer areas, there should be a minimum,
differential, negative pressure of a minimum of 0.01-inch
water column between the buffer area and ante area. Within
the buffer area, the PEC must be 100 percent exhausted (no
recirculation of air) directly to the exterior through a
high-efficiency particulate air (HEPA)-filtered exhaust.
For pharmacies
that are low or medium risk, and do not compound hazardous
drugs, and where the facility has elected not to have a
physical barrier between buffer and ante areas, the
regulations specify that a system of “displacement airflow
shall be employed … (which) utilizes a low pressure
differential, high airflow principle … (which) requires an
air velocity of 40 feet per minute or more from the buffer
area across the line of demarcation into the ante area.”
It should be noted that this HVAC benchmark, which is
required of all “open” USP 797 facilities (i.e.,
facilities that do not have a wall/door between buffer
room and ante room), has been historically difficult to
achieve.
There are
additional HVAC factors to be considered in the facility
design. The first is air changes, where the regulations
specify that there be a minimum of 30 air changes per hour
(ACPH) in an ISO 7 space supplied by HEPA-filtered air.
That number of air changes could be split between the PEC
contributing no more than 15 ACPH and the HEPA-filter
supply air to the area at least 15 ACPH.
The minimum
requirement for ACPH for the buffer area is 30 ACPH and
should be based on compounding activities in the area.
Compounding processes that generate a significant amount
of particles may increase the ACPH requirements. Also, the
regulation proscribes an OR-type, unidirectional airflow
design, where the HEPA-filtered air is distributed from
supply grills in the ceiling and returned through return
grills mounted low in the wall.
Moving on to
the architectural regulations for the buffer area, only
items absolutely essential to compounding should be
brought in. Items like refrigerators, computers and
printers can be placed in a buffer area as long as there
is an accommodation for the particles they may generate.
Typically, the placement of low-wall returns behind these
devices will help sweep away any particles.
Any furniture
or equipment should be “nonpermeable, nonshedding,
cleanable and resistant to disinfectants.” All surfaces
(e.g., ceilings, walls, floors, fixtures, shelving,
counters and cabinets) should be “smooth, impervious, free
from cracks and crevices, and nonshedding.” Junctures of
ceilings to wall “shall be coved or caulked.” Wall
construction can be either epoxy-coated gypsum board, or
heavy gauge polymer panels locked together and sealed. No
sinks or floor drains are allowed in the buffer area.
Work surfaces
should be made of smooth, impervious materials such as
stainless steel or molded plastic. Carts should be made of
stainless steel wire, nonporous plastic or sheet metal
construction. Storage shelving, counters and cabinets
should also be “smooth, impervious, free from cracks and
crevices, nonshedding, cleanable and disinfectable.”
To learn more
This
article provides a general overview on the portions of the
new USP 797 regulation that pertain most to the planning,
design and construction of USP 797-compliant facilities.
General
questions can be addressed to USP at
http://usp.org.
Additionally, a selection of some of the most commonly
asked questions and answers provided by industry experts
also can be accessed at
http://usp797.org/Questions_and_Answers.htm.
William N. Bernstein, ACHA, AIA, is
a principal of Bernstein & Associates Architects in New
York. He can be contacted via his e-mail address at
wb@bernarch.com. |